News
Rebuilding the brain with stem cells: is it a no-brainer?
By Anitta Rose Chacko
Most of us may have experienced feeling on edge whilst playing the classic tumbling tower game of Jenga. Particularly, when it is your turn to remove a block from the tower and place it on the top of what can be described as a progressively unstable structure. More often than not, this feeling swiftly shifts to disappointment when the tower inevitably tumbles over. Nevertheless, we are aware that we can very easily rebuild the tower, either to play again or store away the game for later use. But, have you ever considered that perhaps much like rebuilding a tower for Jenga, the brain too can be rebuilt? Although this idea may seem extremely dubious, many researchers have been focussed on trying to do just this using stem cells.
Parkinson’s disease is a devastating neurodegenerative disorder that progressively affects the brain. This means that cells in particular areas of the brain become increasingly damaged over time, much like the increasing instability of a Jenga tower as blocks are removed successively. The development of Parkinson’s disease has been considered attributable to the lack of dopamine, a chemical which the brain utilises as a messenger to ultimately regulate movement. In individuals with Parkinson’s disease, dopamine producing cells called dopaminergic neurones, which reside in an area called the substantia nigra located in a division of the brain known as the midbrain (Figure 1), are impaired and progressively lost. This results in increasing problems with functions such as movement.

It is therefore logical that replacement of these impaired dopamine producing cells to restore dopamine levels in the brain, stands as a potential therapy for Parkinson’s disease. This is where stem cell therapy comes into play. Researchers have been utilising pluripotent stem cells to generate dopamine producing cells, which can ultimately be transplanted into individuals with Parkinson’s disease to restore dopamine levels and in turn the loss of functions caused by the condition. This is hardly surprising, considering that pluripotent stem cells are cells that have the capacity to indefinitely give rise to themselves and become almost any cell in our bodies.
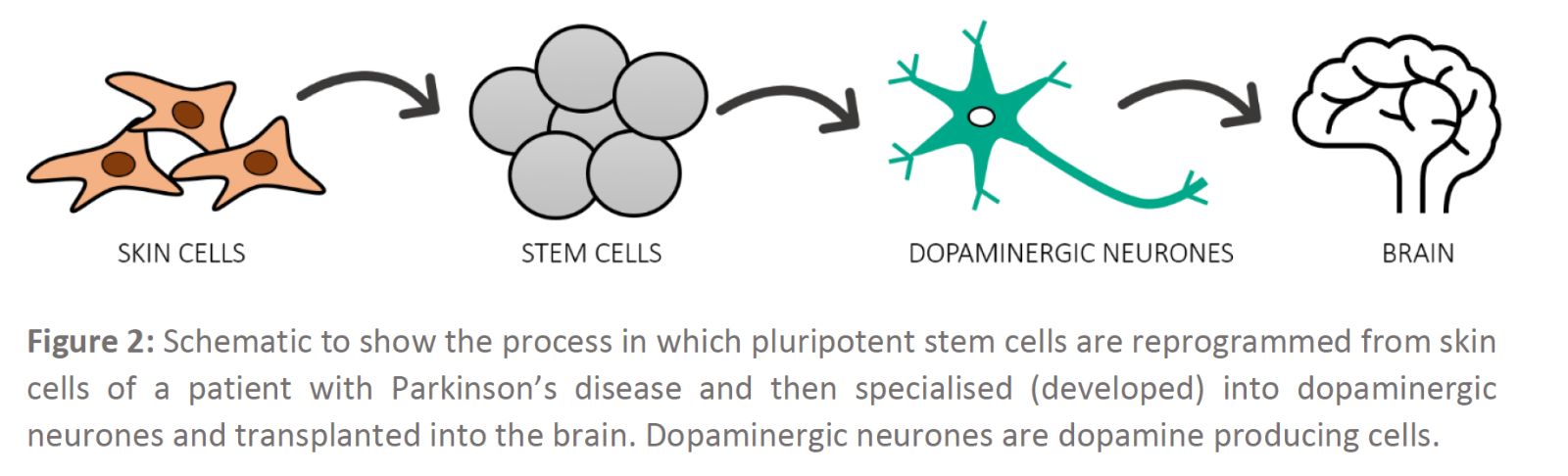
The process of using stem cells to treat or prevent a condition, such as replacing damaged or dead dopaminergic neurones to treat Parkinson’s disease, is known as stem cell therapy. Many researchers have been developing strategies using specific controls and conditions to re-programme skin cells collected from individuals with Parkinson’s disease into pluripotent stem cells and then turn these cells into dopamine producing cells (Figure 2). Essentially, this process can be thought of as perfecting different recipes to make dopamine producing cells, which can then be transferred into a Parkinson’s disease affected brain in a process known as transplantation.
Research focussed on investigating dopaminergic neurones derived from induced pluripotent stem cells (skin cells that have been reprogrammed to become pluripotent stem cells) in a Parkinson’s disease model was published by a group of researchers in Japan. Kikuchi et al observed that dopaminergic neurones derived from induced pluripotent stem cells were able to function and survive as well as improve movement after transplantation studies in primates. Therefore, suggesting that their study is applicable for clinical studies to treat patients with Parkinson’s disease.
With that all being said, the decision to use stem cell therapy to treat Parkinson’s disease should be a no-brainer, right? Well, not quite. The progression of stem cell therapies for Parkinson’s disease has been astounding, but as in the words of Spiderman, “With great power comes great responsibility”. This is because, great responsibility is definitely present regarding stem cell therapies. For example, it is imperative to know whether transplanted stem cell therapies in Parkinson’s individuals will have any detrimental effects in the long term. Will the therapy lead to any cancer formation, how long do transplanted cells remain effective at producing dopamine? These are all very important questions that must be addressed when evaluating potential stem cell therapies for diseases.
Having stringent regulations like we currently have in the UK regarding these potential therapies for Parkinson’s disease are a vital necessity to ensure safety. These measures also benefit the general public as it allows the public to trust in scientific research and remove any misconceptions they may have regarding cell and gene therapy.
Currently in the UK, around 145, 000 individuals are diagnosed with Parkinson’s disease and this figure is expected to rapidly increase in the coming years. Hence, for a devastating condition like this, where everyday activities as simple as writing, walking and smiling can be affected, research on stem cell therapies provides substantial hope and promise for the future. Although it is clearly evident that rebuilding the brain using stem cells will never be as simple as building a tower for Jenga, it does however highlight how scientific advancement in the field of cell and gene therapy have enabled such immense progress to be made with regards to the treatment of disease.
References:
Kikuchi T, Morizane A, Doi D, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017;548(7669):592-596.
Parkinson’s UK Media and Press Office [Internet]. Parkinson’s UK; 2020. [Accessed 09 June 2020]. Available from: https://www.parkinsons.org.uk/
Parmar, M., Grealish, S. & Henchcliffe, C. The future of stem cell therapies for Parkinson disease. Nature Reviews Neuroscience. 2020; 21(1): 103–115.