News
Putting the dopamine back in Parkinson’s
Author: Lucy Lewis, 21st January 2019
One in 37 people in the UK are diagnosed with Parkinson’s disease (PD) during their lifetime (Parkinson’s UK, 2018). Those 2.7% of people have their lives completely changed by a neurodegenerative condition that can make even your closest relative seem like a stranger. Their movements become much slower, they often become rigid and sometimes find it difficult to even initiate a movement, even though they’re really trying to.
It’s not just difficulty with locomotion that troubles them; patients can also suffer from a number of psychiatric disturbances, such as depression, anxiety, hallucinations, and even dementia (NICE, 2006). The exact causes of the psychiatric symptoms are difficult to pinpoint, but we know that the disruptions to movement in PD are likely due to the loss of specific neurons in the brain which produce the chemical dopamine. These are known as dopaminergic neurons, and specifically, those lost in PD connect two parts of the brain known as the Substantia Nigra and the Striatum (Braak et al 2003).
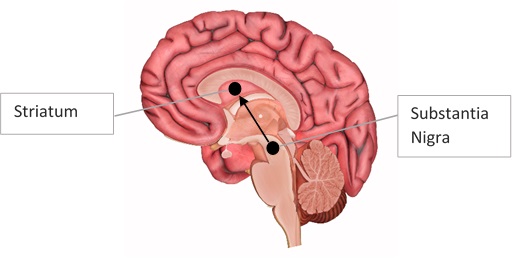
Figure 1. Nigrostriatal pathway. A diagram showing dopamine released from the Substantia Nigra to the Striatum. This is called the ‘nigrostriatal pathway’. Image adapted from: http://www.innerbody.com/image/nerv02.html.
Despite the debilitating nature and high prevalence of PD, we are far from finding a cure. Often patients are given drug treatments containing “L-Dopa”, which is the building block for dopamine that can pass from the body into the brain where it is converted to dopamine (Nagatsu & Sawada 2009). However, it’s very difficult to control how much dopamine is actually released. After a prolonged period of taking these drugs patients develop severe side effects called ‘dyskinesia’, where they experience uncontrolled movement (Thanvi et al 2007). They also become more tolerant to the drug and its effectiveness wears off over time. It’s a frustrating cycle for both researchers and patients, to know the main cause of a disease but to struggle finding a cure.
In recent years, researchers are more hopeful with ongoing developments in cell replacement therapy. This is a type of treatment involving transplantation of stem cells into a target area, for example an area of the nigrostriatal pathway in the case of PD. Early-stage stem cells, including embryonic stem cells, are capable of developing into a number of possible cell types, whereas adult stem cells are restricted to the functions associated with the tissue they are found in.
Various researchers have shown that stem cells taken from human embryos can be transformed into dopamine-producing cells when they are transplanted into rodent brains (Grealish et al 2014). These cells looked like dopaminergic neurons and, most importantly, functioned like them. The rodents used in these studies are models of PD, where their own dopaminergic neurons are damaged and they consequently display motor deficits similar to those seen in PD patients. When transplanted into the nigrostriatal pathway of rodents, stem cell-derived neurons began to integrate and develop into mature, dopamine-producing neurons with long-term survival. As the transplants matured, motor deficits of the rodents progressively improved too.
Another way to generate dopaminergic cells through stem cell technology is by using ‘induced-pluripotent stem cells’ (iPSCs). This technique is quite fascinating, as it involves taking mature cells, such as skin cells, and turning them back into early stage stem cells using a mix of various chemicals and compounds (Yamanaka 2009). These stem cells can then be treated like the embryonic-derived cells, where they are programmed to become dopaminergic cells once transplanted. Having the option of using iPSCs means there are fewer ethical concerns with the reduced use of embryos, but ultimately the competition between the two stem cell technologies is based on their potential for improving patient symptoms.
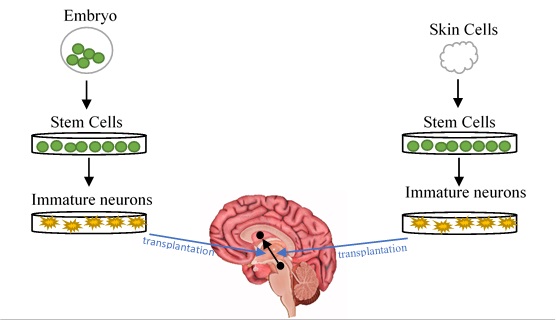
Figure 2. Cell Replacement Therapy. Diagrams to illustrate the process involved in two types of stem cell technology currently researched for treating PD: embryonic-derived stem cells (left) and iPSCs (right).
Preclinical research is abundant at present, with several research groups investigating the potential for their specific cells to survive, function and improve symptoms in animal models. Each group has developed their own method and specific conditions for generating these cells, in the hopes that their cell replacement therapy will be successful in tackling this debilitating disorder.
In terms of clinical research, the first clinical trial to transplant human iPSCs in to PD patients began in October 2018 (Knoepfler 2018). This early in the trial, there is not much to be said for the potential of these transplants to improve quality of life in these patients, but the fact that the trial is underway is a major advancement in PD research. These patients will be followed for a number of months following transplantation, and we can only hope that the trials can progress to investigate the long-term effects of cell replacement therapy in PD.
References
Braak, H., et al. (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging 24:197-211.
Grealish, S., et al. (2014). Human ESC-Derived Dopamine Neurons Show Similar Preclinical Efficacy and Potency to Fetal Neurons when Grafted in a Rat Model of Parkinson’s Disease. Cell Stem Cell 15:653-665.
Knoepfler, P. (2018, November 18). Hope on Parkinson’s front: Japan IPSC trial 1stpatient. The Niche [Blog Post]. Retrieved from: https://ipscell.com/2018/11/hope-on-parkinsons-front-japan-ipsc-trial-1st-patient/
Nagatsu, T. & Sawada, M. (2009). L-Dopa therapy for Parkinson’s disease: Past, present, and future. Parkinsonism and Related Disorders 15:S3-S8.
NICE (2017). Parkinson’s disease: diagnosis and management. Retrieved from: https://www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-4538466253
Parkinson’s UK (2018). The Incidence and Prevalence of Parkinson’s in the UK: Results from the Clinical Practice Research Datalink Summary report. Retrieved from: https://www.parkinsons.org.uk/professionals/resources/incidence-and-prevalence-parkinsons-uk-report
Thanvi, B., et al. (2007). Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgraduate Medical Journal 83:384–388.
Yamanaka, S. (2009). A fresh look at iPS cells. Cell 137:13-17
By Lucy Lewis; PhD Student; Cardiff University