News
Cracking the enthesis code
Author: Subashan Vadibeler, 22nd February 2019
The very thought of an injury is enough to send chills running down the spine of those involved in sports. And we know exactly why this fear exists because we’ve seen countless celebrity sportsmen and sportswomen at their prime succumb to injuries. After all, not everyone can bounce back from major injuries such as a torn shoulder tendon like Maria Sharapova or Kobe Bryant.
In fact, more than three quarters of shoulder repair surgeries fail while long-term disabilities occurring as a direct consequence of sports-related injuries, such as pain and osteoporosis, remain a huge burden (Apostolakos et al, 2014). With advancements in tissue engineering and cell therapy, current treatment limitations are being addressed to formulate a dynamic and personalised solution to treat injured athletes.
If we look back at cases of injuries sustained during sports, it would be immediately clear that some locations in the body are more commonly affected than others. These injury “hotspots” may even show a bias for a type of sport. For instance, tennis players are more likely to sustain an elbow injury, netball players usually suffer from knee injury while a specific type of ring finger injury is more commonly seen among rugby and football players (Ruchelsman et al, 2011). This can be attributed to overuse of a particular muscle tendon (eg. swinging motion in tennis) or a movement in sports that makes the player more vulnerable to injury (eg. pivoting in netball).
However, overuse and sports manoeuvres can’t be the only explanation because even within particular body locations, some muscle tendons can injure more frequently than others in the same area. Let’s take the shoulder for example – both deltoid and rotator cuff muscles reside here (Figure 1) but deltoid tendon injuries are extremely rare while the same can’t be said for the latter. Surely this means that there’s something more to these injury-prone tendons besides the mechanical difference in their use. For that reason, when we consider muscles, bones and tendons as a combined workforce, we should not overlook the importance of another crucial entity – the entheses (singular, enthesis), which are small locations where tendon attaches with bone.
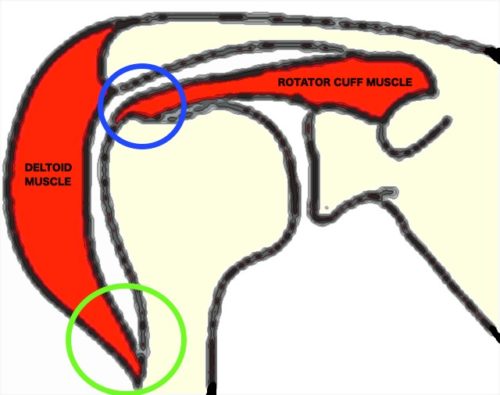
Figure 1. Deltoid and part of rotator cuff muscle are shown. When we lift our arm, the rotator cuff enthesis (blue circle) bends more than the deltoid enthesis (green circle).
Scientists have had an inkling since the late 1920s that not all tendon attachment to bone at the enthesis are as simple as gluing two distinct structures together (1929, cited in Shaw, 2007). This idea challenged the traditional dogma that viewed tendons as ropes of uniform substance which muscles use to grasp onto bones. Instead, it introduced a new perspective of gradation within tendon attachment sites. According to this idea, if we were to zoom into the entheses such as the Achilles tendon insertion at the heel (Figure 2), we would see several zones of cells and its surrounding substance where tendon slowly morphs into bone (Bannerman et al, 2014). During this transition, tendons slowly lose their fibrous quality and their surrounding starts to mimic bone which is enclosed by a calcium-rich substance.
Interestingly, this special transition at the enthesis does not occur for every muscle in the body. Its formation is driven by many external factors such as the amount of force that the muscle generates and how much the attachment site is bent during movement. If we go back to the previous shoulder muscle comparison, this distinctive transition is present at the rotator cuff enthesis but absent for deltoid because the former is bent and manipulated to a greater extent when we lift up our arm (Figure 2). Essentially, the enthesis which acts as a messenger carrying force between muscle and bone is designed to ensure that tendon attachment at vulnerable body locations remain strong and versatile.
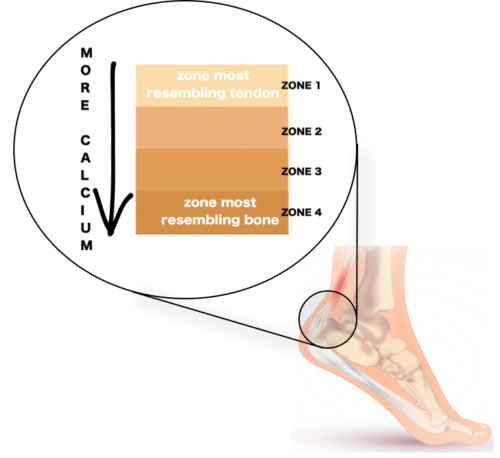
Figure 2. Four zones can be seen if we look closer into the Achilles tendon enthesis.
Although the gradation at entheses may be efficient at preventing injuries, the high failure rate of corrective surgeries can also be attributed to this complex transition which usually does not regenerate after damage. Upon injury, the intricate zones that anchor tendon to bone is instead replaced by haphazard scar tissue that lacks the support that the native gradation once provided. Hence, re-injuries are common when athletes return to the field and begin using their muscles vigorously again.
But what if we can circumvent this hurdle by growing entheses with their intact gradation zones in the laboratory which can then be used to replace patient’s injured tendon insertion? This feat could be achieved by growing replacement graft using stem cells which have the potential to become many cell types including bone, muscle, cartilage and tendon (Paxton et al, 2012). With appropriate signals provided and adequate force applied while growing, these stem cells can be conditioned to form bone and muscle along with the intermediate tendon and its enthesis. With that, the focus of surgery can shift from attempting to repair the delicate tendon attachment to substituting defective attachment sites with tissue engineered replacement graft.
However, the utility of replacement graft formed by tissue engineering remains restricted by our limited knowledge on the anatomical variations of different entheses in the body. For instance, we can’t assume that the attachment site for Achilles tendon is similar to that of the shoulder. In reality, critical qualities of entheses such as the thickness of zones are distinct for different muscles so in-depth studies for each tendon attachment in our body is required to analyse these minute differences that can potentially translate into huge clinical consequences. Besides structural differences, uncertainties in physical and chemical factors influencing attachment site formation are also still present. Thus, we also need to better understand how cells at the enthesis talk to each other and how they react to external forces which can ultimately determine how engineered entheses develop.
In essence, biological, physical and chemical factors governing entheses generation form pieces of an unsolved puzzle which impede the advancement of tissue engineered grafts. With ongoing research, we are slowly fitting together these puzzle pieces with the ultimate aim to crack the enthesis code and unburden athletes from the toll of sports-related injuries.
References
Apostolakos, J., et al. (2014) The enthesis: a review of the tendon-to-bone insertion. Muscles, ligaments and tendons journal. 4(3), 333.
Bannerman, A., et al. (2014) Imaging the hard/soft tissue interface. Biotechnology letters. 36(3), 403-415.
Paxton, J.Z., et al. (2012) Current progress in enthesis repair: strategies for interfacial tissue engineering. Orthopedic Muscul Sys. 1, 003.
Ruchelsman, D.E., et al. (2011) Avulsion injuries of the flexor digitorum profundus tendon. J Am Acad Orthop Surg. 19, 152-62.
Shaw, H.M. (2007) The structure and function of entheses and enthesis organs. PhD thesis, University of Wales, Cardiff, United Kingdom.
By Subashan Vadibeler; Undergraduate Student; University of Malaya