News
Overcoming the disconnection
Author: Beth MacLeod, 1st February 2019
We all have a universe inside our head. A hundred billion neurons making connections with one another, constantly firing and passing messages from our brain, down the spinal cord, and to every part of our body. These messages control each of our senses and every movement we make. But sometimes these connections fail, and the messages never make it to where they are meant to go.
This is what happens in motor neurone disease (MND). The term MND encompasses a range of neurodegenerative diseases which affect the motor neurons that travel to the muscles. These neurons degenerate and the line of communication between brain and muscle breaks down, meaning that the signal never reaches its intended destination. It is a disconnection. Muscles that are not receiving any stimulation will become weak, and if they remain inactive will start to waste. This causes gradual paralysis leading to problems with movement, speech, swallowing and breathing, and eventually death. Patients are often described as being ‘locked in’, unable to speak and unable to move, but their mind and thoughts still free (1,2,3).
The length of time people can live after diagnosis varies. MND proves fatal for more than 50% of people after only two years, but others can live with MND for decades (1,3). Whatever the case, there is no cure and the disease is eventually fatal. At the time of writing only one drug, Riluzole, was approved for treatment in the UK, but on average it may only increase the life span of a patient by a few months (4).
Stem cells – cells which are not yet specialised and so can become any type of cell – have potential in the treatment of neurodegenerative diseases. In the infancy of stem cell research much controversy came from the fact that stem cells used in experiments were embryonic, raising questions about the ethics of using embryos for research. However, the discovery that adult cells can be reprogrammed back into stem cells, creating induced pluripotent stem cells (iPSCs), reduced the need for embryos in research (5).
For treating MND stem cells could potentially be used to replace degenerated motor neurons. This comes with many difficulties however, as a single motor neuron may make contact with several muscle fibres, creating a highly complex circuit and making it extremely challenging to create the right connections.
A different method is using stem cells to promote the survival of cells around them, by production of protective neurotrophic factors that support the growth and survival of neurons, and also modulate the immune response (6,7,8). So far this technique has shown only limited short-term benefits, as the survival of transplanted cells is threatened by the disease environment. If the stem cells are rejected or do not survive then they can’t have any beneficial effect, and so research has been conducted to find techniques that will promote survival of the transplanted stem cells (8).
How to supercharge your stem cells
In MND it is typical for there to be a lack of oxygen to cells, and so one method of preconditioning has been to expose the stem cells to a low oxygen environment before transplantation. Experiments have shown that this causes upregulation of certain genes (such as those that prevent cell death and those that produce the pro-survival neurotrophic factors) leading to enhanced survival after transplantation and increased protective properties. Rodent studies have shown a greater improvement in motor functions when they are treated with pre-conditioned, rather than untreated, stem cells (8,9,10).
Chemical compounds can also be used to precondition stem cells. Similar to above this leads to upregulation of neurotrophic genes and growth factors which reduces death of transplanted cells and promotes functional recovery in experimental models of the disease (8,11). This method shows very few safety concerns making it highly attractive for further testing in human clinical trials.
Genetic engineering is another possible method that could be utilised to increase the production of neurotrophic and other protective factors. Overexpressing the genes for these factors would create stem cells that should show better survival as well as promoting growth and survival of the cells around them. They will have greater neuroprotective properties and show a stronger therapeutic effect in models of MND (12).
Another technique to promote survival is to add a scaffold that supports the stem cells, with the intention of reducing stress on the cells and creating a more favourable environment for them. Scaffolds can be built using molecules similar to those in the extracellular matrix, a natural scaffold found between cells that provides structure and support to the cells surrounding it. Cells with this type of scaffold have been shown to have a survival rate which is five times higher than untreated cells, strongly suggesting that this method would greatly increase transplanted stem cell survival (8).
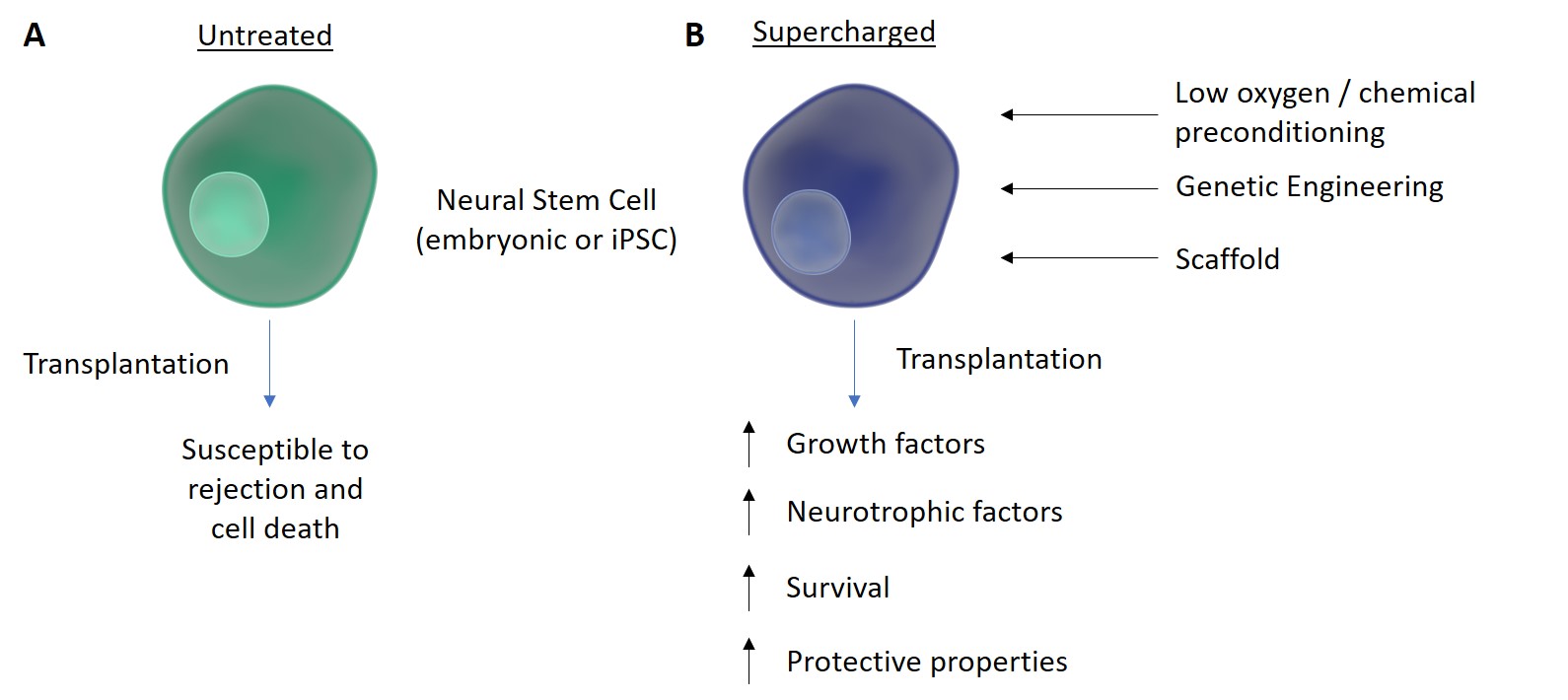
Figure 1. Supercharging Stem Cells. Neural stem cells can be used in experiments and can either be embryonic or induced pluripotent stem cells (iPSCs) that have been reprogrammed from adult cells. A) The use of these stem cells as a treatment for MND is limited by rejection and cell death caused by the disease environment. B) Increased survival of transplanted stem cells is seen when they are preconditioned using a variety of techniques. Preconditioning with a low oxygen concentration or with chemicals, genetic engineering, and adding a supporting scaffold have been shown to promote survival and release of protective factors. This suggests that supercharging stem cells in this way could be a step towards using cell therapy as a treatment for MND.Adapted from http://www.somersault1824.com/science-illustrations/
The research into these techniques is still in the early stages, but they each show great potential for enhancing the survival of transplanted stem cells, as well as promoting their neuroprotective and supportive effects. Development of a therapy for treatment of MND and other neurological diseases is still far away, but the potential is there, and more studies should be conducted to gain further understanding of the methods discussed here. In the future stem cell transplants may be able to overcome the disconnection found in MND. Not by replacing motor neurons and rebuilding the circuit, but by protecting those that already exist.
References
1) MNDA. Last revised 2018. What is MND? [ONLINE] Available at:
https://www.mndassociation.org/about-mnd/where-do-i-start/what-is-mnd/
[Accessed 26 August 2018]
2) MNDA. Last revised 2018. Introduction to MND research. [ONLINE] Available at: https://www.mndassociation.org/wp-content/uploads/A-Overview-of-MND-research.pdf
[Accessed 26 August 2018]
3) NHS. Last revised 2018. Motor neurone disease. [ONLINE] Available at:
https://www.nhs.uk/conditions/motor-neurone-disease/
[Accessed 26 August 2018]
4) MNDA. 2015. Riluzole. [ONLINE] Available at:
https://www.mndassociation.org/wp-content/uploads/2015/07/05a-riluzole.pdf
[Accessed 26 August 2018]
5) MNDA. Last revised 2018. Stem Cells and MND. [ONLINE] Available at:
https://www.mndassociation.org/wp-content/uploads/2013/10/F-Stem-Cells.pdf
[Accessed 27 August 2018]
6) Nizzardo M, et al., (2014) Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 23:342–354
7) Faravelli I, et al., (2014) Stem cell transplantation for amyotrophic lateral sclerosis: therapeutic potential and perspectives on clinical translation. Cell Mol Life Sci 71:3257–3268
8) Abati E, et al., (2018). Preconditoning and Cellular Engineering to Increase the Survival of Transplanted Neural Stem Cells for Motor Neuron Disease Therapy. Mol Neurobiol 1-12
9) Theus MH, et al., (2008) In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol 210:656–670.
10) Wei L, et al., (2012) Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis 46:635–645.
11) Sakata H, et al., (2012) Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 32:3462–3473
12) Thomsen GM, et al., (2018) Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cells 36:1122–1131
By Beth MacLeod; Undergraduate Student; University of Dundee