News
A final chance to breathe: Could gene therapy cure cystic fibrosis lung disease?
Author: Alexandra McCarron, 14th March 2019
Imagine a newborn baby. She looks perfectly healthy on the outside, but on the inside, her DNA contains one simple spelling error that causes a disease called cystic fibrosis (CF). From the day of diagnosis and for the rest of her life onwards, she may need to take a collection of daily medications to try slow an unstoppable disease. As she grows-up, her lungs will gradually become diseased. It will start with lung infections, progressive lung damage, and worsening problems with cough and breathlessness. It will inevitably end with irreparable lung damage and at some stage the need for a lung transplant. While she might be lucky enough to one day receive a life-saving lung transplant, it’s more likely that she will die waiting. Now imagine if we could offer this child and all people with CF a future where their lungs are cured.
We know that CF arises when a baby receives two faulty copies of a gene called CFTR. This gene and its protein product are responsible for salt transport across certain barriers in the human body (Collawn et al. 2014). Although the CFTR protein performs a seemingly trivial task, when salt transport is disturbed, it has dire consequences for many organs. The most serious consequence is the build-up of thick, sticky mucus that clogs the lungs and leads to life-threatening lung infections (Boucher 2002).
For decades, scientists and doctors have worked tirelessly towards developing effective treatments for CF. Although modern medicine has dramatically improved average life expectancy, which is now in the 40’s, many patients still won’t survive their teenage years (National Guideline 2017). Not only does CF reduce lifespan, it also impacts significantly on quality of life. CF treatments are life consuming. They consist of daily lung-physiotherapy, nebulised medications, and a suite of oral drugs including intensive antibiotic regimes. Some patients take up to 50 tablets a day, yet still require frequent hospitalisation.
Could we cure CF lung disease?
Effective treatment of CF lung disease will undoubtedly improve and lengthen the lives of patients. One possible approach is to use gene therapy. The idea of CF gene therapy is to deliver healthy copies of the CFTR gene to the airway cells. Functioning CFTR protein is then produced in the patients’ lungs, normal salt transport is restored, and disease progression is halted (Ziady et al. 2006). If gene therapy is given early in life, before the disease takes hold, there is potential to prevent or even cure CF lung disease.
So how do you get the CFTR gene into the airway cells?
One way is to use an engineered virus. Over millions of years, viruses have become experts at delivering their genetic material into cells. By removing most of the viral genes, we can transform a virus from super-pathogen to tiny gene delivery vehicle. We can also use a special category of viruses (retroviruses) that “stitch” the therapeutic gene into our chromosomes. This means that the gene permanently becomes part of the treated cells’ genome and the benefit will last much longer.
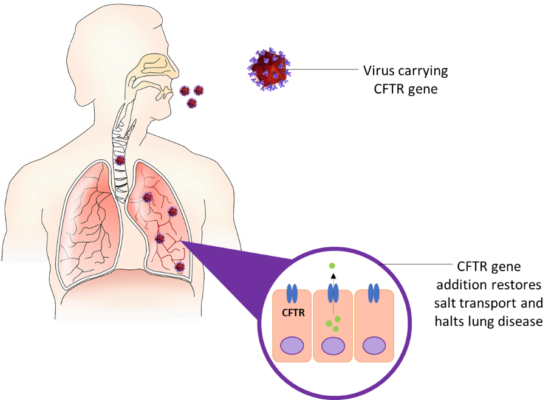
What challenges does CF gene therapy face?
Even though CF lung disease was one of the first conceived targets for gene therapy over 20 years ago, making it a reality has proven challenging. Firstly, the lungs are a difficult target. Our airways have evolved barriers to prevent viruses and other nasty pathogens from being able to enter. A problem given our therapy is based on a virus. For gene therapy to be safe and effective, the viral vehicle must evade the body’s natural surveillance system. Early gene therapy trials have taught us that immune responses can cause serious illness and in rare cases, death (Lehrman 1999). Therefore, we must carefully design and test gene therapies to avoid immune issues.
Safety concerns can arise when using retroviruses. We know these viruses are good for gene therapy because they provide long-lasting benefit by stitching the gene into a person’s DNA. But what if the virus stitches itself into the wrong location? Unfortunately, in one of the first gene therapy clinical trials this is exactly what happened, and the result was that a small number of patients developed cancer (Hacein-Bey-Abina et al. 2008). Nowadays, the viruses we use for gene therapy are much safer and the risk of cancer is very low, but it’s still a serious concern that must be monitored closely (Neil 2017).
Gene therapies are also very costly. Developing a gene therapy product is unappealing to most big pharmaceutical companies as it can take years for return on investment, and a treatment marketed as once-off or intermittent is not as profitable as medications that are taken daily. Manufacturing gene therapy products is also expensive. For gene therapies to become mainstream, cheaper, larger and more efficient production processes are needed. Currently marketed gene therapies for other conditions can cost anywhere between $500,000 – $1 million US dollars per person (Hanna et al. 2017). For most people this once-off expense is unaffordable, so until costs are substantially reduced, or government subsidies are introduced, these treatments will be unattainable for those who so desperately need them.
Should we be hopeful for the future?
The gene therapy field is gaining momentum. Several gene therapy products are already on the market, with more on the way. Once a theory, gene therapy is now becoming a reality for patients suffering incurable diseases. Treated individuals with an inherited form of anaemia no longer require blood transfusions to survive (Cavazzana et al. 2015); children with severe immune deficiency, so called “bubble kids”, can now safely live outside the constrains of a plastic bubble (Aiuti et al. 2017); cancer patients have gone into remission (Zhao et al. 2018); Parkinson’s disease progression has been slowed (Axelsen et al. 2018); and people with hereditary blindness have been able to see again (Russell et al. 2017).
Labs around the world investigating virus-based gene therapy for CF lung disease have already shown promising results. We are now closer than ever-before, with a Phase I clinical trial planned to commence soon in the UK (Alton et al. 2017). We still have a way to go before gene therapy becomes part of mainstream medicine, but with rapidly advancing research and growing investment in genetic therapies, we should remain optimistic that the future of CF is one with lung gene therapy.
References
Aiuti, A., et al. (2017). “Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products.” EMBO molecular medicine 9(6): 737-740.
Alton, E. W. F. W., et al. (2017). “Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis.” Thorax 72(2): 137-147.
Axelsen, T. M., et al. (2018). “Gene Therapy for Parkinson’s Disease, An Update.”Journal of Parkinson’s disease 8(2): 195-215.
Boucher, R. C. (2002). “An overview of the pathogenesis of cystic fibrosis lung disease.” Adv Drug Deliv Rev 54(11): 1359-1371.
Cavazzana, M., et al. (2015). “Outcomes of Gene Therapy for Severe Sickle Disease and Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex Vivo with a Lentiviral Beta AT87Q-Globin Vector.” Blood 126(23): 202.
Collawn, J. F., et al. (2014). “CFTR and lung homeostasis.” Am J Physiol Lung Cell Mol Physiol 307(12): 917-923.
Hacein-Bey-Abina, S., et al. (2008). “Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1.” The Journal of clinical investigation118(9): 3132-3142.
Hanna, E., et al. (2017). “Gene therapies development: slow progress and promising prospect.” J Mark Access Health Policy 5(1): 1265293.
Lehrman, S. (1999). “Virus treatment questioned after gene therapy death.” Nature401(6753): 517-518.
National Institute for Health and Care Excellence (2017) Clinical Guidelines. Cystic Fibrosis: Diagnosis and management. London, National Institute for Health and Care Excellence (UK). https://www.nice.org.uk/guidance/ng78
Neil, J. C. (2017). “Safety of Retroviral Vectors in Clinical Applications: Lessons from Retroviral Biology and Pathogenesis.” eLS.
Russell, S., et al. (2017). “Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial.” The Lancet 390(10097): 849-860.
Zhao, Z., et al. (2018). “The application of CAR-T cell therapy in hematological malignancies: advantages and challenges.“ Acta Pharmaceutica Sinica B 8(4): 539-551.
Ziady, A. G., et al. (2006). “Current prospects for gene therapy of cystic fibrosis.“ Curr Opin Pharmacol 6(5): 515-521.
By Alexandra McCarron; PhD student; Cystic Fibrosis Airway Research Group, University of Adelaide.